Nanoscale Forces
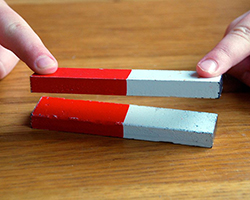
You pick up a magnet in each hand and hold them so the bottom of each is facing the other. You move them closer. As you try to push the magnets together, you can feel a force pushing them apart instead. These magnets have two different sides, with different poles. Similar poles will repel each other, but if you flip one over, so that a north and south pole are close to one another, the magnets pull together and connect.
One of the forces that controls the assembly of nanostructures is very similar. Electrostatic forces are the attractive/repulsive forces when parts of the molecules act like magnets. Many molecules used in bionanotechnology are charged (either positively or negatively). These types of forces are important when designing a structure.
Like Oil and Water
The other forces involved are called hydrophobic forces. Hydrophobic force is the attraction between two “fatty” molecules or parts of a molecule. You can try this at home: take a glass of water, and add a few drops of oil to it. Move the water around very gently and, after a while, you should find that most of the oil drops have gathered into one or two larger floating oil drops. What is happening here?
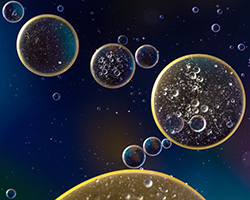
Well, fatty molecules don’t like to interact with water. (Have you ever heard the term “like oil and water”? They don’t mix.) Because the fatty molecules want to get away from water, when they encounter other fatty molecules, they will come together to form a fatty “pocket”. This lets more of the fatty molecules get farther away from the water.
This hydrophobic force or effect is one of the main driving forces for self-assembly. The hydrophobic effect allows the self-assembled structure to be at its most stable state. The most stable state of a biomolecules is usually a position in which the molecule is the most “relaxed.” This means there isn’t stress or strain on the bonds within the molecule, trying to get it to change shape. So, it can hold that state easily, making it stable.
To build at a nanoscale, you need to understand electrostatic and hydrophobic forces in detail. With this knowledge, you can better predict the self-assembly of structures.
Day gecko image by Anrita1705 via Pixabay.
Read more about: Nanobiotechnology: Nature's Tiny Machines
Bibliographic details:
- Article: Nanoscale Forces
- Author(s): Dr. Biology
- Publisher: Arizona State University School of Life Sciences Ask A Biologist
- Site name: ASU - Ask A Biologist
- Date published:
- Date accessed:
- Link: https://askabiologist.asu.edu/nanoscale-forces
APA Style
Dr. Biology. (). Nanoscale Forces. ASU - Ask A Biologist. Retrieved from https://askabiologist.asu.edu/nanoscale-forces
Chicago Manual of Style
Dr. Biology. "Nanoscale Forces". ASU - Ask A Biologist. . https://askabiologist.asu.edu/nanoscale-forces
Dr. Biology. "Nanoscale Forces". ASU - Ask A Biologist. . ASU - Ask A Biologist, Web. https://askabiologist.asu.edu/nanoscale-forces
MLA 2017 Style

Be Part of
Ask A Biologist
By volunteering, or simply sending us feedback on the site. Scientists, teachers, writers, illustrators, and translators are all important to the program. If you are interested in helping with the website we have a Volunteers page to get the process started.